Shaky Ground for VNDA: FDA Denies Approval for Tradipitant
Vanda Pharmaceuticals Inc. announced a setback as the FDA rejected its new drug application for tradipitant, a potential treatment for gastroparesis. Struggling to overcome the blow, VNDA shares plummeted by 6.1% after the news hit the market.
The shadow of gastroparesis looms large – a condition causing delayed gastric emptying, which has lacked a new treatment in over four decades. This denial has left VNDA investors reeling, impacting the company’s year-to-date performance, which had shown promise with a 10.2% increase compared to the industry’s meager 0.4% rise.
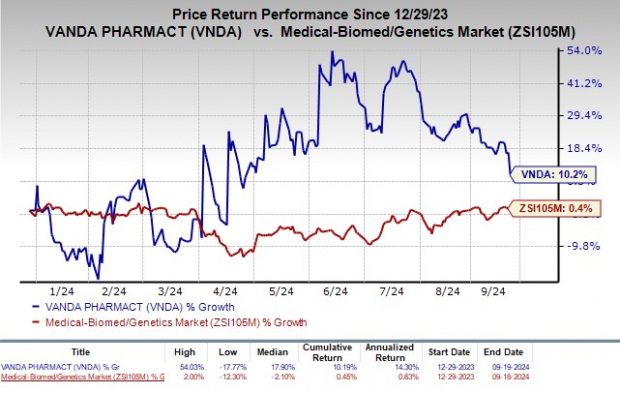
Image Source: Zacks Investment Research
Reading Between the Lines of FDA’s Complete Response Letter
The FDA’s complete response letter detailed a discordant demand for further studies on tradipitant, not aligning with expert advice in the field. Going against the tides of scientific understanding, the FDA’s decision came after a prolonged wait of more than 185 days, violating the statutory timeline set by the Food Drug and Cosmetic Act.
Vanda’s consistent appeals for an advisory committee meeting fell on deaf ears, leaving the company in a precarious situation. Desperate patients have resorted to a Citizen Petition, imploring the FDA to reconsider its stance on tradipitant.
Bouncing back: VNDA’s Resilient Path Forward
Despite the setback, Vanda remains undeterred in its pursuit of bringing tradipitant to market for gastroparesis. Not putting all its eggs in one basket, the company is concurrently exploring the drug’s potential for preventing motion-sickness-induced vomiting. Positivity echoed in the corridors of VNDA with promising outcomes from a phase III study for the latter indication, culminating in plans to submit an NDA for motion sickness treatment in late 2024.
Stock Landscape: A Dive into the Deep Waters
Investors eyeing the biotech sector may seek solace in other opportunities as Vanda grapples with this setback. Illumina, Krystal Biotech, and Fulcrum Therapeutics present themselves as appealing alternatives, all currently flaunting a Zacks Rank #1 (Strong Buy).
While Illumina showcases resilience with an upward trajectory in earnings estimates and a string of earnings surprises, Krystal Biotech stands out with a steady earnings track record and positive forecasts. On the other hand, Fulcrum Therapeutics exhibits a narrowing loss per share, despite its rocky year-to-date performance.
In the realm of biotech, amid VNDA’s turbulence, these stocks offer a glimmer of hope, serving as potential safe harbors in a sea of uncertainty.
